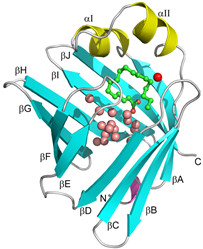
Structure of human heart-type fatty acid-binding protein (FABP3).
FABP3 has a clum-like shell structure which consists of a pair of five-stranded antiparallel β-sheets, called β-barrel (cyan), that surround a large water-filled cavity covered on the top by two short parallel α-helices (yellow). The cavity accommodates about 13 water molecules (pink and red spheres) and a fatty acid (green ball and stick model).
Proteins Recognize the Various Lengths of Fatty Acids Using a Water Cluster
Fatty acid-binding proteins (FABPs) are small proteins for cytosolic delivery of fatty acids (FAs), which are used as fuels for aerobic exercise and/or raw materials for inflammatory mediators. Assoc. Prof. Shigeru Matsuoka, Assoc. Prof. Shigeru Sugiyama, and Prof. Michio Murata at Osaka University revealed that human heart-type FABP (FABP3) identifies FAs not by exact matching but by “fuzzy recognition” of fundamental structural similarities among numerous FAs, where two different type of water clusters are involved in measuring the length of string-like molecule FAs. This finding sheds light on molecular mechanism of a promiscuous lipid-protein interaction that would play key roles in FA-related diseases, such as metabolic syndrome and schizophrenic. The research was published online in the internationally leading chemistry journal, Angewandte Chemie International Edition, on December 9, 2014.
(Link) http://resou.osaka-u.ac.jp/en/research/2015/20150107_1









