Fig. A schematic illustration of post-synthetic creation of lanthanide hydroxide cluster in the host crystals.
The easy way to get a square deal
Researchers at Osaka University have discovered a new method to easily add lanthanide cubanes into a previously synthesized metallo-supramolecular framework. By simply soaking a crystal in a cubane-containing solution, the molecules become intercalated via a single-crystal-to-single-crystal transformation. This research may help chemists design cost-effective methods of storing energy or develop new catalysts.
Once thought too unstable to exist, cubanes are synthetic molecules with atoms arranged to form a cube. The extremely strained right-angle bonds store a great deal of chemical energy, like tightly wound springs. However, once created by chemists, cubanes can keep their shape for a long time. These properties make cubanes attractive candidates for storing energy, as well as for accelerating reactions as catalysts.
Now, scientists at Osaka University have shown how to immobilize cubanes made from lanthanide elements into crystal frameworks. “We discovered a facile synthetic method for lanthanide hydroxide clusters, which may be used as functional materials,” says first author Nobuto Yoshinari.
Lanthanides are a group of chemically similar elements, known to many people for their special section on most versions of the periodic table. The researchers found that by immersing a crystal of K6[Rh4Zn4O(L-cys)12] in a solution containing Ln4(OH)4 (lanthanide hydroxide) cubane molecules, the cubane molecules became attached inside the empty pockets of the crystal without disrupting its structure. A metal-organic framework (MOF) was obtained when using the heavier lanthanides, while the lighter elements yielded ionic solid structures.
“Previously, lanthanide hydroxide clusters have been synthesized under harsh conditions, but our new method requires only the soaking of the host crystals into a lanthanide salt solution at room temperature,” senior author Takumi Konno explains.
The team tested the catalytic activity of the frameworks when speeding up a hydrolysis reaction. They found that the effectiveness of the catalyst depended on which element from the lanthanide series was used. The researchers also measured the magnetic susceptibility of the metal ions inside the frameworks, and found a cooling effect when exposed to a decreasing magnetic fields. The lanthanide hydroxide clusters created inside the crystal framework are expected to be useful for a wide range of applications, including materials for magnetic cooling as well as advanced heterogeneous catalysts.
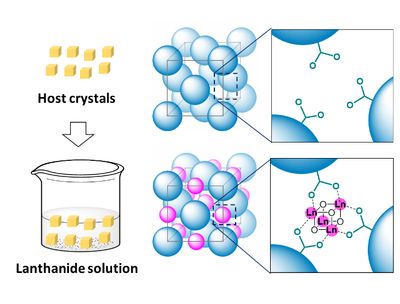
The article, “Single-crystal-to-single-crystal installation of Ln4(OH)4 cubanes in an anionic metallosupramolecular framework,” was published in Angewandte Chemie International Edition at DOI: https://doi.org/10.1002/anie.202008296.
Related links









