Figure
Mechanism for FliL, a component protein of the flagellar motor, clarified
A team of researchers from Osaka University and Nagoya University clarified the detailed structure and function of FliL, a stomatin-like protein that assists the bacterial flagellar motor function; that is, how FliL regulates membrane proteins, or ion channels.
Bacteria swim in the water by rotating their helical flagellar filament like a screw for propulsion. There is a small motor made of protein with a diameter of about 45 nm at the base of the flagellum. Rotation of bacterial flagellar motors is driven by an influx of ions (H+, Na+) from the outside to the inside of a cell with notably high driving energy efficiency.
Torque of the flagellar motor is generated by the interaction between two components: the rotor and the stator. When ions flow inside the stator, rotor-stator interaction changes, generating high-speed rotational speed (about 300 ~ 1,000 rotations/second).
Bacteria move in highly viscous environments, such as mucus and the cellular surface of hosts, causing infections. Under such high-load conditions, flagellar motors need to enhance motor performance more than during swimming, requiring FLiL proteins.
Because loss of FliL due to genetic mutation leads many bacteria to lose their ability to move during adaptation to the host habitat, an infection does not occur. How FliL enhances the motor performance and how FliL senses high-load conditions such as the highly viscous environments were unknown.
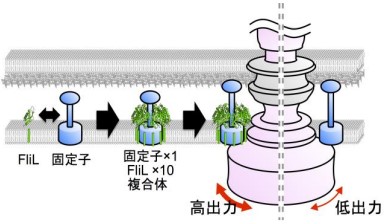
In this study, the team focused on a key protein, FliL of Vibrio alginolyticus, especially, FliLC, a core region of the periplasmic region of FliL (FliLPeri). Using X-ray diffraction data at synchrotron beamline BL41XU in the large synchrotron radiation facility SPring-8 (Harima, Japan), they clarified the molecular mechanism of Vibrio FliL:
- FliL forms a ring assembly consisting of 10 molecules and the inner wall of the FliL ring interacts with the stator to regulate stator function.
- FliL has a striking structural similarity to a conserved domain of stomatin, which is involved in ion channel regulation in some organisms, including mammals.
- A FliL ring forms a complex with the stator unit, a membrane protein, and FliL modulates stator function in the bacterial flagellar motor
The findings in this study will lead to the clarification of mechanisms behind the enhancement of bacterial motor performance and the regulation of action of membrane proteins.
The Article, “Structure of Vibrio FliL, a new stomatin-like protein that assists the bacterial flagellar motor function” was published in mBio at DOI: https://doi.org/10.1128/mBio.00292-19.
Related links
Laboratory of Meacromolecular Structure, Department of Macromolecular Science, Graduate School of Science, Osaka University (link in Japanese)









