Figure 1: Structure of the newly developed ionic crystal. The pathway in which the ions can travel is highlighted in yellow.
(credit: Osaka University)
Building Better Batteries by Borrowing from Biology
A research team at Osaka University has reported a new advance in the design of materials for use in rechargeable batteries, under high humidity conditions. Using inspiration from living cells that can block smaller particles but let larger particles pass through, the researchers were able to create a material with highly mobile potassium ions that can easily migrate in response to electric fields. This work may help make rechargeable batteries safe and inexpensive enough to drastically reduce the cost of electric cars and portable consumer electronics.
Rechargeable lithium-ion batteries are widely used in laptops, cell phones, and even electric and hybrid cars. Unfortunately, these batteries are expensive, and have even been known to burst into flames on occasion. New materials that do not use lithium could reduce the cost and improve the safety of these batteries, and have the potential to greatly accelerate the adoption of energy-efficient electric cars. Both sodium and potassium ions are potential candidates that can be used to replace lithium, as they are cheap and in high supply. However, sodium and potassium ions are much larger ions than lithium, so they move sluggishly through most materials. These positive ions are further slowed by the strong attractive forces to the negative charges in crystalline materials. “Potassium ions possess low mobility in the solid state due to their large size, which is a disadvantage for constructing batteries,” explains corresponding author Takumi Konno.
To solve this problem, the researchers used the same mechanism your cells employ to allow the large potassium ions to pass through their membranes while simultaneously keeping out smaller particles. Living systems achieve this seemingly impossible feat by considering not just the ion themselves, but also the surrounding water molecules, called the “hydration layer,” that are attracted to the ion’s positive charge. In fact, the smaller the ion, the larger and more tightly bound its associated hydration layer will be. Specialized potassium channels in cell membranes are just the right size to allow hydrated potassium ions to pass through, but block the large hydration layers of smaller ions.
The researchers developed an ionic crystal using rhodium, zinc, and oxygen atoms. Just as with the selective biological channels, the mobility of the ions in the crystal was found to be higher for the bigger potassium ions, compared with the smaller lithium ions. In fact, the potassium ions moved so easily, the crystal was classified as a “superionic conductor.” The researchers found that the current material had the largest hydrated potassium ion mobility ever seen to date.
“Remarkably, the crystal exhibited a particularly high ion conductivity due to the fast migration of hydrated potassium ions in the crystal lattice” lead author Nobuto Yoshinari says. “Such superionic conductivity of hydrated potassium ions in the solid state is unprecedented, and may lead to both safer and cheaper rechargeable batteries.”
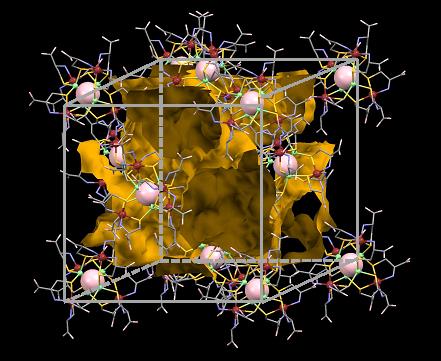
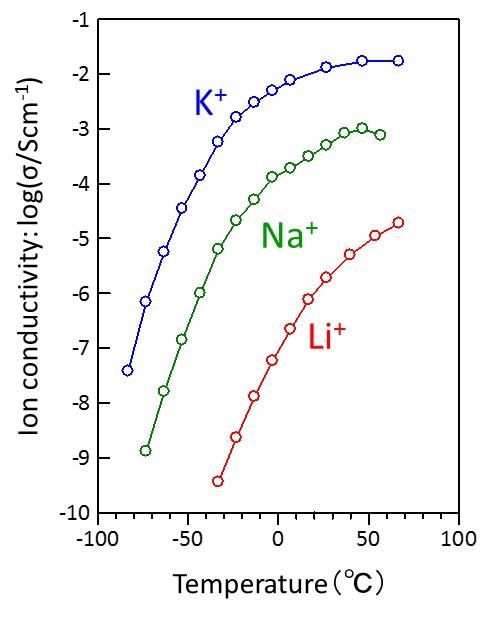
Figure 2: Conductivities of lithium (Li+, red), sodium (Na+, green), and potassium (K+, blue) ions inside the crystal at different temperatures. The conductivities increase even as the sizes of the ions increase.
(credit: Osaka University)
This work “Mobility of hydrated alkali metal ions in metallosupramolecular ionic crystals” was published in Chemical Science at DOI: https://doi.org/10.1039/c8sc04204g.









